Infection Control
Healthcare-associated infections (HAIs) account for an additional $28 to $33 billion in healthcare costs each year. Approximately 1.7 million infections and 99,000 associated deaths occurred in 2002.The Centers for Disease Control's (CDC) identifies the problem of antimicrobial resistance as an emerging threat to world health; cites the cost of hospital-acquired infections "caused by just six common kinds of resistant bacteria" to be "at least $1.3 billion per year."
ICET has identified a clear need to develop bio polymeric interfacial surfaces which would:
- Prevent the adherence of bacteria
- Prevent calcium and magnesium salt deposits on material.
 ICET has addressed these and developed highly lubricious and efficacious surface coating formulations with antimicrobial properties. Such formulations are coatable on silicone, polyurethane, PVC, metals and latex devices.
ICET has addressed these and developed highly lubricious and efficacious surface coating formulations with antimicrobial properties. Such formulations are coatable on silicone, polyurethane, PVC, metals and latex devices.
 The coating process is simple such as dip coating and a few milligrams of the coating could provide active protection. Ready to coat or custom formulations can be provided.
The coating process is simple such as dip coating and a few milligrams of the coating could provide active protection. Ready to coat or custom formulations can be provided.
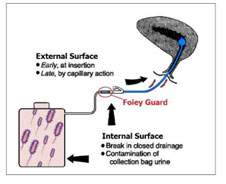 ICET-TIC: Foley Catheter System
ICET-TIC: Foley Catheter System
Clinically poor performance of existing antimicrobial catheters in the market is now well recognized and there is a dire need for infection control urinary catheters. An FDA approved
ICET’s commercial Silicone Foley Catheter is coated on internal &
Clinically poor performance of existing antimicrobial catheters in the market is now well recognized and there is a dire need for infection control urinary catheters. An FDA approved ICET’s commercial Silicone Foley Catheter is coated on internal & external surfaces including the
balloon that release soluble silver antimicrobial up to 14 days for reducing infections.
The accessory (Foley Guard TM) connected to the catheter outlet is outside the body, which releases copper and silver in a soluble form. This is designed to block ascending
infections as colonized urine collection systems constitute a source of potentially resistant organisms.
 This photo shows bacterial biofilm growth on Bardex silver hydrogel ( Yellow) vs ICET catheter
With the end of reimbursement for costs associated with catheter associated urinary tract infection (CAUTI), hospitals are eager to prevent or reduce their incidence of this condition and are examining their urinary catheter management practices. Evidence based guidelines are available but are not universally followed.
This photo shows bacterial biofilm growth on Bardex silver hydrogel ( Yellow) vs ICET catheter
With the end of reimbursement for costs associated with catheter associated urinary tract infection (CAUTI), hospitals are eager to prevent or reduce their incidence of this condition and are examining their urinary catheter management practices. Evidence based guidelines are available but are not universally followed.
Antimicrobial and Therapeutics Releasing Platform Technology
 Compounding Technologies
Compounding Technologies
ICET can provide its custom antimicrobial formulations for compounding with certain medical resins for dry extrusion processes. ICET active formula could be compounded with medical or consumer grade resins and extruded to a desired shape. The temperature limitations for this process are 300 F. Currently an extruded antimicrobial catheter accessory device, Foley Guard connected to the ICET antimicrobial catheter was tested in a clinical trial. This has been fully qualified for biocompatibility and functionality and ICET has
obtained an Investigational Device Exemption form the FDA.
ICET Link : Antimicrobial Technology for Food and Beverage Packaging
ICET formulations coated catheter with repeated challenges of microbes provide potent reductions in microbial growth over a period of several days.
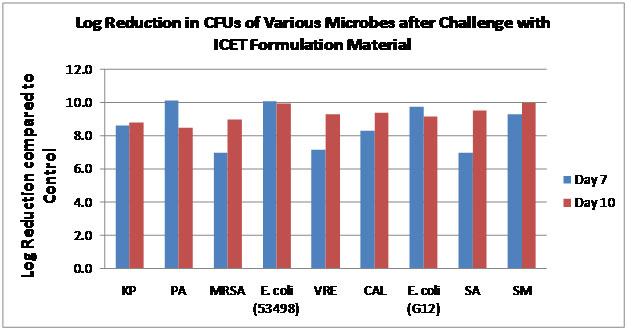
Graph depicts the log reduction in microbial growth as compared to uncoated controls. ICET formulations are challenged with 106 CFUs/mL twice during over a period of 10 days. The two challenges occur before the 7th day. Plating results indicate at least 99.999% reduction in microbial growth as compared to the control (not shown) for days 7 (blue bars) and 10 (red bars). Organisms tested from left to right, Klebsiellapneumoniae, Pseudomonasaeruginosa, Staphylococcus aureus (MRSA), Escherichia coli, Enterococcus faecalis (VRE), Candida albicans, Staphylococcus aureus, and Serratiamarcescens. All organisms are urological clinical isolate.
Log Reduction of Microbes in the Contact Solution Compared to a Control and after Seven Days Incubation.
 Assay Criteria : At least 4 logs reduction (99.99%) of growth (CFUs/mL) in the Foleyguard sample to the control. Organisms from left to right: Klebsiellapneumoniae, Pseudomonas aeruginosa, Staphylococcus aureus(MRSA), Escherchia coli, Enterococcus faecalis(VRE), Candida albicans,E. coli, S. aureus, Serratiamarcesscens, Proteus mirabilis.
Assay Criteria : At least 4 logs reduction (99.99%) of growth (CFUs/mL) in the Foleyguard sample to the control. Organisms from left to right: Klebsiellapneumoniae, Pseudomonas aeruginosa, Staphylococcus aureus(MRSA), Escherchia coli, Enterococcus faecalis(VRE), Candida albicans,E. coli, S. aureus, Serratiamarcesscens, Proteus mirabilis.
- Four US Patents secured from 1999- 2007; one international including India.
- Catheter accessory first generation prototype made : 2002-2003
- Pilot production & clinical finished by 2005
ICET completed an initial Human clinical trials on an add-on antimicrobialdevice accessory only connected to a commercial the Foley catheter. Conducted at U. Wisconsin Hospitals under the direction of Dennis Maki M.D, a world renowned Infectious disease specialist.
Clinical data Showed 50% Reduction of all Intraluminal Infections; had no effect on extraluminal infections brought in by a commercial Foley catheter.
ShanthaSarangapani *, Katherine Cavedon, David Gage Journal of Biomedical Materials Research, Volume 29 Issue 10, Pages 1185 - 1191,1995
Therefore ICET Foley Catheter coatings were developed &qualified for biocompatibility per ISO 10993.



